What is rotavirus disease?
Worldwide, rotaviruses are the commonest cause of community-acquired gastroenteritis in children.
WHO estimates in 2013 about 215,000 children aged <5 years die each year from rotavirus infections the vast majority of these children live in low-resource countries and over two million children are hospitalised each year with pronounced dehydration. Approximately 37 percent of childhood diarrhoeal deaths and 3.4 percent of all deaths in children under five are due to rotavirus infection. Children under five years of age, especially those between 6 months and two years are most vulnerable to the disease. Death from rotavirus infection is very rare in countries where there is ready access to oral and parenteral rehydration.
Rotavirus infection is the most common cause of gastroenteritis in children in Ireland under the age of 5 years. It is most common in the Spring and Winter.
Most children will recover at home but some need to be admitted to hospital. Every year in Ireland almost 1000 children under the age of 5 are admitted to hospital with rotavirus infection. The average length of time they spend in hospital is 5 days. Infants and young children can now be protected from this disease by the rotavirus oral vaccine.
Back to top
How safe is rotavirus oral vaccine?
Rotavirus oral vaccine has been shown to be very safe and well tolerated by the majority of infants. Over 300 million doses have been distributed worldwide.
Back to top
How do people get rotavirus disease?
Rotavirus is very infectious and can spread easily. It can be spread through hand to mouth contact, such as from touching toys, surfaces, dirty nappies or can be spread through the air from coughing and sneezing.
Back to top
How many cases of rotavirus occur in Ireland?
In Ireland, from 2006-2015 inclusive there was an average of 2,400 cases of rotavirus notified each year in the 0-4 years age group (see figure below). Most of these cases are seen in the less than1 year age group (see figure below).
Figure: Number of rotavirus notifications for 0-4 years, 2006-2015
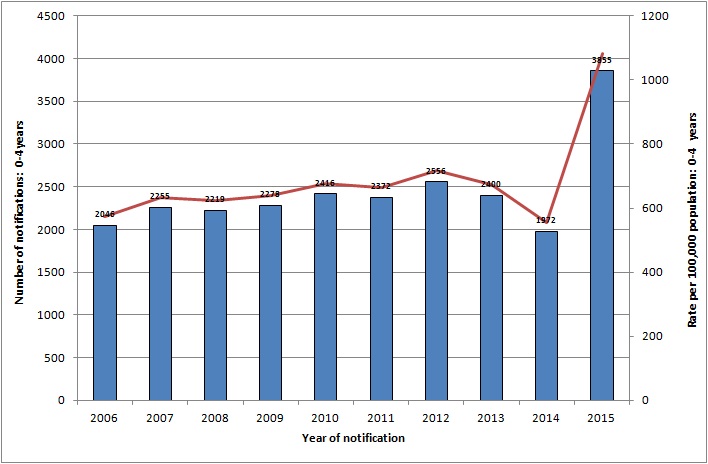
Source HPSC
Figure: Age specific incidence rate per 100,000 population of notified rotavirus cases
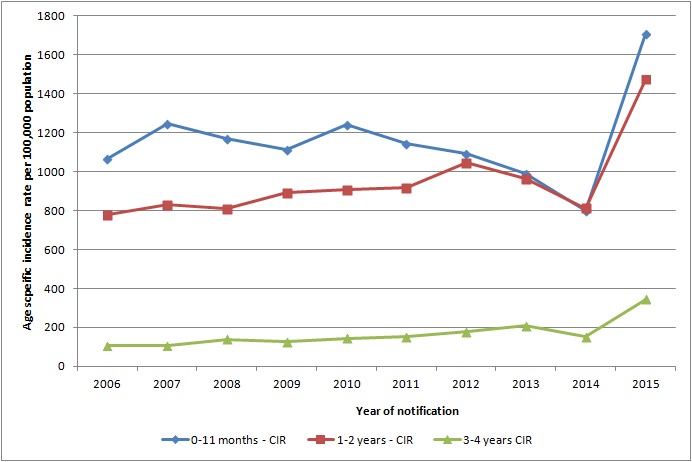
Source HPSC
Back to top
Is the rotavirus vaccine reducing the incidence of rotavirus disease?
With the introduction of rotavirus vaccine to the primary immunisation schedule for all infants born on or after 1st October 2016, the number of cases of rotavirus infection reported in Ireland has fallen from 2305 cases in 2017 to 636 cases in 2018 (72%).
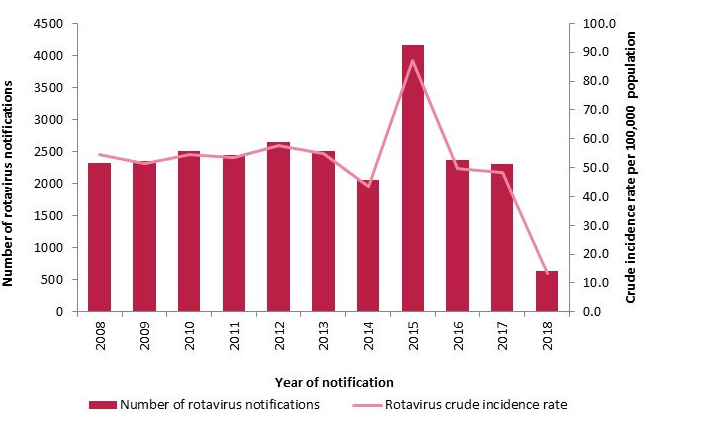
Source: HPSC
Back to top
What are the symptoms of rotavirus disease?
Rotavirus causes diarrhoea which can be severe, stomach cramps, vomiting, dehydration and a low-grade fever. Symptoms occur 1 – 3 days after being exposed to rotavirus infection and can last approximately 3-8 days. Rarely diarrhoea may last for up to 3 weeks. Children with rotavirus disease can spread the infection from 2 days before they become unwell with the infection and up to 10 days after they become unwell.
If 1,000 children get rotavirus:
- 1,000 get vomiting and diarrhoea
- 3 will need to be admitted to hospital for treatment.
Young children need to stay in hospital for an average of 5 days, if they are admitted.
Children may get rotavirus disease more than once because there are many different rotavirus types, but second infections tend to be less severe than the first infections.
Many cases of Rotavirus are not notified. This is because children who have Rotavirus may recover at home, or are looked after by their GP without being tested for the virus. The real number of cases of Rotavirus is likely to be higher.
If you are concerned your infant may have rotavirus infection, please attend your GP for review.
Back to top
Who should get rotavirus oral vaccine?
Rotavirus disease is prevented by vaccination. All children born on or after 1 October 2016 are given rotavirus oral vaccine at 2 and 4 months of age.
Rotavirus oral vaccine should not be given to infants who are 8 months 0 days and older.
Back to top
Are there any reasons why rotavirus oral vaccine should not be given?
Contraindications
- Anaphylactic reaction to a previous dose of the vaccine
- Anaphylactic reaction to any constituent of the vaccine
- A previous history of intussusception
- Severe Combined Immunodeficiency Disorder (SCID)
- A malformation of the gastrointestinal tract which might predispose them to intussusception
- A hereditary fructose intolerance, sucrose-isomaltase deficiency or glucose-galactose malabsorption
- Aged 8 months and 0 days and older.
Back to top
Why is the rotavirus vaccine recommended up to 8 months and 0 days only?
The SPC for Rotarix advises
“The (Rotarix) vaccination course should preferably be given before 16 weeks of age, but must be completed by the age of 24 week”.
However the National Immunisation Advisory Committee (NIAC) recommend
“If an infant is late presenting for vaccination, they can receive their first dose of vaccine up until the age of 7 months and 0 days (Rotarix). The final dose can then be given before 8 months and 0 days”.
The National Immunisation Advisory Committee (NIAC) recommends that to provide maximum protection the first oral rotavirus vaccine (Rotarix) dose should be given by 15 weeks however if an infant presents later than 15 weeks of age the infant can still receive the 1st dose of the rotavirus vaccine up to the age of 7 months and 0 days and receive the second dose before 8 months and 0 days.
Each chapter of the immunisation guidelines states:
“In some circumstances, advice in these guidelines may differ from that in the Summary of Product Characteristics of the vaccines. When this occurs, the recommendations in these guidelines, which are based on current expert advice from NIAC, should be followed”.
Back to top
What is SCID and why is it relevant to rotavirus oral vaccine?
SCID is a rare inherited primary immune deficiency characterised by severe impairment in T-cell development and function. In Ireland, 1 case is diagnosed every year. It is more common in infants in some ethnic groups.
The risk of an Irish infant being born with SCID is approximately 1:70,000. The risk for some ethnic groups, including Irish Travellers can be higher. Children affected by SCID can also become ill from live vaccines, including rotavirus oral vaccine. These vaccines contain viruses that are attenuated (weakened) and do not harm children with a healthy immune system. In children with SCID however, these attenuated viruses and bacteria may cause severe, life-threatening infections.
However the risk from rotavirus vaccine needs to be balanced against the risk of an infant with undiagnosed SCID contracting rotavirus disease.
Back to top
How to check if the infant is at risk of SCID and what to do if they are?
Ask the infant's parent / caregiver the following questions:
Are there any diseases in the infant’s family that affect the immune system?
Did anyone in either family need a bone marrow transplant as a infant?
If the parent/caregiver answers “No” to these questions rotavirus oral vaccine should be given.
If the parent / caregiver answers “Yes” to either of these questions
- check if a Full Blood Count was taken at birth and confirm the results.
if a FBC was not taken, a full blood count with differential white cell, including lymphocyte count should be ordered.
If the lymphocyte count is below <2.0/109 litre referral to a Paediatrician should be made urgently.
Any infant at risk of SCID should NOT be given rotavirus oral vaccine.
Precautions
Vaccination should be delayed until recovery for infants who are suffering from
- an acute febrile illness
- an acute vomiting or diarrhoea illness
Back to top
What is rotavirus oral vaccine?
Rotavirus oral vaccine is a live attenuated viral vaccine. There are two vaccines licensed for use in Ireland, one is a two dose schedule (Rotarix, GSK) and the other a three dose schedule (Rotateq, MSD Ireland (Human Health)).
The rotavirus vaccine being used as part of the HSE programme is Rotarix (GSK).
Back to top
What does rotavirus oral vaccine protect against?
Rotavirus oral vaccine protects against gastrointestinal diseases caused by rotavirus infection. It will not prevent against diseases caused by other gastrointestinal viral infections, such as norovirus.
Back to top
How effective is rotavirus oral vaccine?
Rotavirus oral vaccine is very effective in preventing rotavirus disease in young infants. Since the vaccine was introduced into the immunisation schedule in Australia, they have seen a 70% decline in hospitalisations due to rotavirus gastroenteritis in the under-five year’s age group. Preliminary laboratory data from the UK has also identified a 70% reduction in rotavirus reports since the vaccine programme was implemented there. A European study identified a reduction in hospitalisations from rotavirus infection of between 65 to 84%. It estimated the vaccine effectiveness at between 82-94%.
Back to top
What is in rotavirus oral vaccine?
Rotavirus oral vaccine is formulated in a sugary solution.
There is a plunger stopper and a protective tip cap as part of the vaccine kit. These are made of rubber butyl and should not affect those with a latex allergy. The vaccine is not formulated in eggs and therefore should not affect those with an egg allergy.
A full list of vaccine constituents can be found at www.hpra.ie
Back to top
Are other countries vaccinating against rotavirus?
Yes, the World Health Organization has recommended rotavirus vaccination and many other countries are providing the vaccine to their citizens. The vaccine was licensed globally in 2006. Over 300 million doses have been distributed worldwide. Rotavirus was added to the immunisation schedule in Australia in 2007. The UK implemented a national programme in 2013. The USA and 11 other EU countries have also implemented rotavirus vaccination programmes.
Back to top
Should a infant who has already been infected with rotavirus still get the vaccine?
Yes. Infants who have recovered from a rotavirus infection may not be immune to all of the virus types present in the vaccine. The vaccine will help better protect the infant against further episodes of infection. So infants who have previously had rotavirus disease should still get the vaccines before age 8 months and 0 days
Back to top
How is the vaccine presented?
The vaccine comes in a box containing 10 individual vaccine doses.
The vaccine comes as a small (1.5 mls) oral suspension in a pre-filled oral applicator.
Each individual vaccine dose is ready to use and does not require any reconstitution
Back to top
How should the vaccine appear?
The vaccine dose should be a clear, colourless liquid with no visible particles within it. If any particulate matter is seen in the vaccine, or the vaccine appears anything other than a clear colourless liquid, the vaccine should be clearly marked ‘not for use’, boxed and returned to the HSE National Cold Chain Service (NCCS) when you receive your next delivery.
Back to top
When should rotavirus oral vaccine be given?
The vaccine should be given at the beginning of the visit, while the infant is still content, and before administering injections. This is because the infant is likely to be most settled at the start of the vaccination schedule, which makes administering it easier. Also, the vaccine is within a sugary solution which is helpful in providing pain relief for the remaining vaccines
Back to top
How should the vaccine be given?
The vaccine is an ORAL vaccine.
STEP 1: Remove protective tip cap from the oral applicator (see diagram)
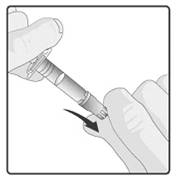
STEP 2: Ensure the infant is sitting in a reclining position.
STEP 3: Insert syringe tip into the infant’s mouth, towards the inner cheek
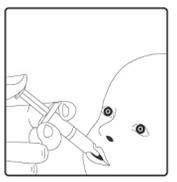
STEP 4: Administer vaccine into the infant’s mouth. The syringe containing the vaccine should be aimed down one side and towards the back of the infant’s mouth. The syringe should not be inserted so far back that the infant gags. All the syringe contents should be given to the infant.
Back to top
What if an infant vomits part or all of a dose of rotavirus vaccine?
If it is judged that more rotavirus vaccine was swallowed than spat out a replacement dose of rotavirus vaccine is not necessary. If vomiting was more than 10 minutes after the vaccine was given a replacement dose of rotavirus vaccine is not necessary.
If a further dose of rotavirus vaccine is necessary the rotavirus vaccine can be given on the same day or it can be given again at any time interval after the dose that was spat out. If the infant vomits out this further dose of rotavirus vaccine there is no need to attempt to give the infant any further dose of the rotavirus vaccine.
The second scheduled rotavirus vaccine can be given at two months after the first dose, usually at four months of age.
Back to top
How soon before or after the vaccine can a infant feed?
A infant can feed at any stage before or after the vaccine.
Back to top
What is the minimum age a infant can receive rotavirus oral vaccine?
The rotavirus vaccine can be given at any time from 6 weeks. However, the best time to receive the first dose is at 2 months of age
Back to top
What is the minimum interval between dose one and dose two of rotavirus vaccine?
The minimum interval between doses of rotavirus vaccine is 4 weeks. The recommended interval is 2 months.
Back to top
What is the maximum interval between doses of rotavirus vaccine?
There is no maximum interval. However, no rotavirus vaccine should be given to a infant after 8 months and 0 days.
Back to top
What if a infant is late presenting for the second dose of vaccine?
Infants can be given their second rotavirus vaccine as long as they are less than 8 months and 0 days, and there is a minimum of 4 weeks since the first dose of rotavirus vaccine.
If a infant is 8 months and 0 days of age or older then they should NOT receive any dose of rotavirus oral vaccine.
These recommendations are in line with the CDC recommendations in the USA.
Back to top
Why should infants not be given rotavirus after 8 months and 0 days?
Infants are most at risk of rotavirus infections and its complications, and get best protection from the rotavirus vaccine, if they receive the vaccine at the scheduled 2 and 4 month visits. However, if they are late presenting they should still be offered the vaccine up until 8 months and 0 days. After this they should not receive the vaccine due to the slight elevated risk identified with intussusception and older infants.
Back to top
What are the side effects of rotavirus oral vaccine?
The vaccine has a very good safety profile. The following are side effects noted with this vaccine and available in the PIL.
Common (1 in 10 infants)
Uncommon (1 in 100 infants)
- Abdominal pain / Flatulence
- Dermatitis (skin inflammation)
Very rare (1 in 50,000)
- Intussusception*
- Blood in stools
- Apnoeas in very premature babies born at or before 28 weeks gestation
- Gastroenteritis in infants with SCID
After rotavirus vaccination, always remind parents to seek medical attention if their infant develops symptoms of intussusception.
Back to top
Does the rotavirus vaccine cause Kawasaki disease?
There is no scientific evidence the rotavirus vaccine causes Kawasaki disease.
Back to top
Is prolonged diarrhoea a side effect of the rotavirus vaccine?
In the studies of the Rotarix vaccine, infants developing diarrhoea was one the most common non serious adverse events for withdrawal from the studies however there was found to be no significant difference between the Rotarix and placebo groups for diarrhoea occurring after vaccination. Diarrhoea after the rotavirus vaccine should only be temporary and last a few days.
Back to top
Is lactose intolerance a side effect of rotavirus vaccine?
Lactose intolerance is a disorder characterised by a lack of the enzyme lactase which normally breaks down the sugar in milk known as lactose, into simpler sugars. Rotarix contains sucrose and sorbitol as excipients and does not contain lactose. Therefore the vaccine could not cause lactose intolerance to develop. Lactose Intolerance is not described as a side effect of Rotarix vaccine.
Back to top
What is intussusception?
Intussusception is a condition where an infant can get a blockage in the bowel. In Ireland, approximately 1 in 1500 infants will get this condition naturally, and it is most common between the ages of 5 months and 1 year. It is thought that for every 100,000 first doses of rotavirus vaccine given, approximately two extra cases of intussusception may be seen. For Ireland, this may mean extra 1-2 cases per year of intussusception which would be related to the rotavirus vaccine. Observational safety studies indicate the increased risk of intussusception is mostly within 7 days after rotavirus vaccination.
Back to top
How would I know if an infant is developing intussusception?
The main symptom of intussusception is severe abdominal pain that comes and goes. Each episode tends to last two to three minutes and the infant may draw their legs up because of the pain. In between episodes, the infant will look very pale, tired and floppy. After 12 hours or so, the pain becomes more constant and the infant will usually go off food, may vomit and have blood in their stools.
Treatment after urgent referral to hospital usually involves a non-operative procedure but on some occasions, an operation is required.
Back to top
Should a infant with a history of intussusception in a sibling have the rotavirus oral vaccine?
Intussusceptions may be idiopathic or associated with underlying diseases.
In those with idiopathic intussusception, familial occurrence is rare however all siblings of an infant who has had an intussusception are at a slightly higher risk of having an intussusception themselves. Familial occurrence of intussusception as a complication of recognisable familial underlying diseases, such as cystic fibrosis, has been reported.
The NIAC immunisation guidelines do not include a family history of intussusception in a sibling as a contraindication or a precaution to receiving the rotavirus oral vaccine.
Back to top
What if a infant was given a dose of vaccine after 8 months and 0 days?
If a infant receives rotavirus oral vaccine on or after 8 months and 0 days no specific clinical monitoring needs to be performed for the infant. However, the parents should be clear regarding the symptoms of intussusception and the need to seek medical help if concerned.
Back to top
Can the rotavirus vaccine be given after 4 months?
If an infant is late presenting for vaccination, they can receive their first dose of vaccine up until the age of 7 months and 0 days (for Rotarix). The final dose can then be given before 8 months and 0 days”. The minimum recommended interval between two doses of rotavirus vaccine is 4 weeks
Back to top
Can one dose of rotavirus vaccine be given?
If an infant is late presenting for rotavirus oral vaccine, then they can receive their first dose of vaccine anytime up to the age of 8 months.
The dose must be given before the child is aged 8 months and 0 days.
In clinical trials the effectiveness after one dose of rotavirus vaccine ranged from 51% to 60%.
Back to top
What if a infant has received another live vaccine within the past four weeks?
There is no minimum interval of time between other live vaccines and oral rotavirus vaccine. The rotavirus vaccine can be given at any time before or after another live vaccine.
Back to top
Can an infant born prematurely >28 weeks gestation have rotavirus vaccine?
Pre-term infants can be given the rotavirus vaccine at 2 and 4 months of age, so long as they have no other contraindications to the vaccine. Preterm infants born 28 weeks of gestation who are vaccinated while in hospital should have respiratory monitoring for 48-72 hours when given their first immunisations, particularly those with a previous history of respiratory immaturity. If the infant has apnoea, bradycardia or desaturations after the first routine immunisation, the second immunisation should also be given in hospital, with respiratory monitoring for 48-72 hours. If the infant has been discharged no respiratory monitoring is necessary. Infants vaccinated whilst in hospital do not need to be isolated from other infants. As the live attenuated vaccine virus can be excreted from the infant for at least 14 days, standard infection control precautions should be followed to reduce the risk of transmission, until the vaccinated infant has been discharged.
Back to top
Can rotavirus vaccine be given to an infant who received Palivizumab (Synagis)?
Palivizumab is a monoclonal antibody and is usually given to prevent serious infection caused by respiratory syncytial virus during the respiratory syncytial virus season. There is however no evidence that Palivizumab interferes with any vaccination and so rotavirus vaccine can be given to a child who has had or is due to receive Palivizumab.
Back to top
Can an infant born prematurely at < 37 weeks gestation have rotavirus vaccine?
Preterm infants are at increased risk for hospitalisation from rotavirus gastroenteritis during the first two years of life. In clinical trials, rotavirus vaccine was generally well tolerated in preterm infants, although relatively small numbers were evaluated. The benefits of rotavirus vaccination of preterm infants outweigh the risk of adverse events.
Back to top
If an infant has a history of adoption or oocyte donation is rotavirus vaccine contraindicated?
It is very unlikely that a person with Severe Combined Immunodeficiency Disorder, hereditary fructose intolerance, sucrose-isomaltase deficiency or glucose-galactose malabsorption would donate an oocyte or sperm and if they did the information about the condition would be made known to the recipient. A history of oocyte/sperm donation or adoption is not a contraindication to the rotavirus vaccine.
Back to top
What if a infant has already had a confirmed rotavirus vaccine infection?
Even if the infant has had a confirmed rotavirus infection, they are still recommended to receive the vaccine. This is to help better protect against further episodes of infection.
Back to top
Can children with an umbilical or inguinal hernia have rotavirus vaccine?
An uncorrected or corrected umbilical or inguinal hernia are not identified as contraindications to rotavirus vaccine immunisation.
Back to top
Can an infant who has mild diarrhoea have the rotavirus vaccine?
Infants with mild acute gastroenteritis can be vaccinated with rotavirus vaccine, particularly if the delay in vaccination might make the infant ineligible to receive the vaccine, even though the immunogenicity and efficacy of the vaccine could be reduced. However if an infant has moderate or severe vomiting or diarrhoea – the rotavirus vaccine should be deferred until the infant recovers. This is to ensure that the vaccine is not passed through the intestines too quickly, which could reduce the effectiveness of the vaccine.
Back to top
What if a infant has a feeding tube in place?
Children with nasogastric feeding tubes should be given the vaccine orally, unless it is absolutely necessary to give it via the tube. Administration of rotavirus vaccine via gastrostomy is acceptable. There is no issue flushing the tube after the vaccine has been administered.
Back to top
What if a infant is on anti-reflux medication?
Infants who are required to take anti-reflux medication should still receive the vaccine. The vaccine itself contains antacid to protect it from the acidic stomach environment and therefore the immune response to the vaccine should not be affected by anti-reflux medication.
Back to top
Are there minimum intervals between administration of rotavirus vaccine and blood transfusion or immunoglobulin product?
No minimum interval is required between administration of a rotavirus vaccine and a blood transfusion or immunoglobulin product.
Back to top
Is a history of positive Coombs test a contraindication to rotavirus oral vaccine?
A history of a positive Coombs test disease is not a contraindication to the rotavirus oral vaccine.
Back to top
Why do I need to ask about diseases in the infant’s family that affect the immune system?
This question is asked to discover if there is a family history of immunodeficiency diagnosed in early childhood requiring bone marrow transplant.
A family history of immunosuppression due to other diseases e.g. carcinoma or treatment is not a contraindication to rotavirus vaccine.
Back to top
What if a infant has an immunodeficiency other than SCID?
Little safety or efficacy data are available following administration of rotavirus vaccine to other infants who are immunocompromised or potentially immunocompromised. Therefore, although rotavirus vaccine strains are considerably attenuated, their administration to infants with known or suspected immunodeficiency other than SCID should be based on careful consideration of potential benefits and risks. HIV positive infants and those of unknown HIV status should receive the rotavirus vaccine.
Back to top
What if a infant is HIV positive?
HIV positive infants and those of unknown HIV status should receive rotavirus vaccine.
Back to top
What if a household member of the infants’ family is immunosuppressed (e.g. undergoing cancer treatment)?
The vaccine virus could be transmitted from the infant to severely immunocompromised contacts through faecal material for at least 14 days. However, vaccination of the infant will offer protection to household contacts from wild-type rotavirus disease and this benefit outweighs any risk from transmission of vaccine virus to immunocompromised close contacts. All members of the household should maintain careful hygiene when changing an infant’s nappy.
Back to top
Is a family history of autoimmune disease a contraindication to the rotavirus oral vaccine?
The question “Are there any diseases in the infant’s family that affect the immune system?” is being asked to establish specifically if there is a family history of Severe Combined Immunodeficiency Disorder - a severe immunodeficiency (where the immune system has lost the ability to fight off infection) rather than a family history of autoimmune disease (where the immune system attacks normal tissues but still has the ability to fight infection). Therefore a family history of autoimmune disease is not a contraindication to rotavirus vaccination.
There is no need to check an FBC.
Back to top
Can rotavirus vaccine be given if the infant’s mother was on immunomodulatory treatment while pregnant?
The National Immunisation Advisory Committee (NIAC) advice on rotavirus vaccine and maternal immunomodulatory treatment was updated in July 2018. The guidelines now advise:
“Infants of women treated with corticosteroids in pregnancy or corticosteroids and low dose methotrexate can receive rotavirus vaccine. However if immunosuppression is anticipated to be moderate or severe, rotavirus vaccine should be deferred until the infant is 4 and 6 months of age. Moderate or major immunosuppression may occur in mothers with severe rheumatoid arthritis or inflammatory bowel disease receiving bDMARDs, and in renal transplant recipients. If in doubt, consult the supervising specialist”.
Immunomodulatory bDMARDs include:
Azathioprine, cyclophosphamide, cyclosporine, hydroxychloroquine, leflunomide, methotrexate, mycophenolic acid preparations, sirolimus and tacrolimus.
Back to top
Can an infant go swimming after rotavirus oral vaccine?
Yes. You should take the usual hygiene measures when changing your infant’s nappy after rotavirus oral vaccine. If your infant gets diarrhoea as a side effect of the vaccine, your infant should not go swimming for two weeks after the diarrhoea has settled.
Back to top
Where can I find out more?
You can ask for further information regarding immunisation from your G.P., Public Health Nurse or local health office.
In addition the links below provide some more detailed information:
Back to top
This page was updated on 1 May 2020