Comments from Expert Advisory Committee and Key Points
- Patients presenting with respiratory symptoms should be managed according to the Acute Respiratory Infections guidance. See the HPSC website for further information on Acute Respiratory Infection - Health Protection Surveillance Centre (hpsc.ie).
- The expert advisory group recommend that the Guidance on the use of antiviral agents for the treatment and prophylaxis of influenza is consulted prior to making the final prescribing decision. It contains FAQ section and more detailed advice on various subgroups below.
- Two antiviral medications are recommended for use in Ireland during the influenza season: oral oseltamivir and inhaled zanamivir. They are both antiviral neuraminidase inhibitors which have activity against seasonal influenza A and B.
- Early antiviral treatment (within 36 to 48 hours of symptom onset) can reduce the risk of complications from influenza, e.g. otitis media in young children, pneumonia and respiratory failure, shorten duration of illness among acutely ill patients and reduce morbidity, including hospitalisation, and mortality among patients with severe infection.
- When influenza is circulating in the community, antiviral therapy should be considered for influenza-like illness in patients who are very ill or who are in recognised risk groups for severe influenza. Information will be issued to GPs when influenza rates increase and antivirals are recommended.
- Uncomplicated influenza: Influenza presenting with fever, cough, sore throat, coryza, generalised symptoms (headache, malaise, myalgia, arthralgia), and sometimes gastrointestinal symptoms, but without any complications of influenza e.g. pneumonia, acute respiratory distress syndrome (ARDS).
- Complicated influenza: Influenza requiring hospital admission and/or with symptoms and signs of lower respiratory tract infection (hypoxaemia, dyspnoea, lung infiltrate), central nervous system involvement and/or a significant exacerbation of an underlying medical condition.
- Previously healthy people (excluding pregnant women) do not need antiviral treatment unless the clinician feels the patient is very ill or is at serious risk of developing complications from influenza. Symptomatic treatment is the preferred option. If you decide to treat, use oseltamivir (PO).
- At risk population: prescribe oseltamivir (PO). Do not wait for laboratory confirmation. Treatment should be started as soon as possible, ideally within 48 hours of onset. There is evidence that treatment may reduce the risk of mortality if commenced up to five days after symptom onset.
- Pregnancy: Antivirals are recommended for pregnant women due to the adverse clinical outcomes that have been observed for influenza in this group. Oseltamivir remains the first line option for the vast majority of pregnant women with influenza, including during seasons that are dominated by influenza A (H1N1)
- Severely immunosuppressed patients: prescribe oseltamivir (PO). Rapid emergence of oseltamivir resistance on treatment has been described in these patients and they should be monitored closely. Some influenza strains have a higher risk for developing oseltamivir resistance, for example, influenza A(H1N1)pdm09. However, resistance is uncommon.
- Clinicians may consider the use of zanamivir (authorised for use in the EU but not marketed in Ireland; zanamivir inhaler is only available as an unlicensed product in Ireland) as first line therapy in immunosuppressed patients with suspected or confirmed influenza A (H1N1) pdm09 subtype based on clinical judgement.
- Suspected or confirmed oseltamivir resistant influenza in a patient who requires treatment: zanamivir (authorised for use in the EU but not marketed in Ireland; zanamivir inhaler is only available as an unlicensed product in Ireland) 10 mg (2 inhalations) every 12 hours. Treatment should be started as soon as possible and ideally within 36 hours for children and within 48 hours for adults of symptom onset.
- Expert advisory group recommend seeking expert advice if considering switch to zanamivir in immunocompromised patients.
- Duration of treatment is usually 5 days (10 days for oseltamivir in immunocompromised adults and adolescents)
- Patients who remain unwell after 5 days antiviral treatment need careful clinical assessment. They may have a secondary bacterial infection, or be infected with an antiviral-resistant influenza strain. Specialist consultation is advised especially in immunocompromised or multi-morbidity. There is no evidence that prolonged courses ( > 5 days) of antiviral treatment improve outcome in non-immunocompromised patients.
Treatment
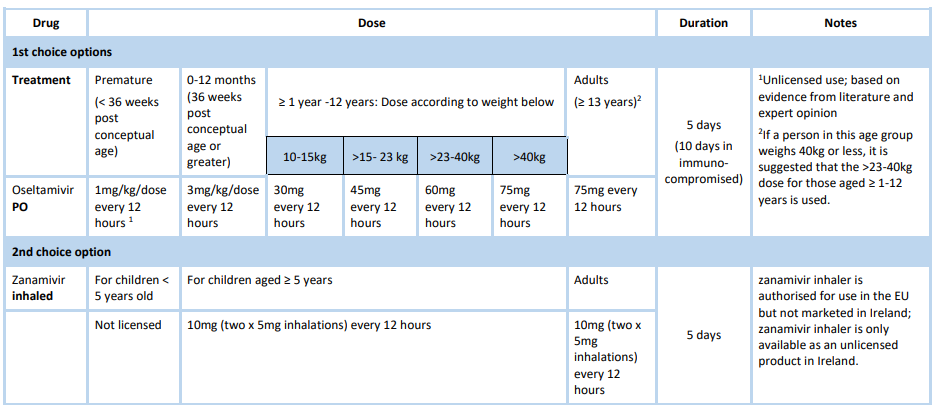
For further information on the treatment of severely immunocompromised patients consult the Antiviral Treatment and Prophylaxis Guidance.
Available products
Oseltamivir
- Oseltamivir capsules (30mg, 45mg and 75mg), oseltamivir powder for oral suspension 6mg/ml.
- Oseltamivir oral suspension should be used for children and adults with swallowing difficulties.
- The SmPC contains further information on extemporaneous formulation of a suspension from the capsules if oral suspension is not available.
- Product information for oseltamivir is available on the HPRA website
- Dose reductions required in renal impairment.
- Refer to Antiviral Treatment and Prophylaxis Guidance for further information on dosing in haemo-dialysis, peritoneal dialysis and haemo(dia)filtration.
Zanamivir
- Zanamivir powder for inhalation 5mg per dose. It should be noted that zanamivir inhaler is authorised for use in the EU but not marketed in Ireland; zanamivir inhaler is only available as an unlicensed product in Ireland.
- Product information is available on the HPRA website.
- Zanamivir inhaled, warning not to be nebulised
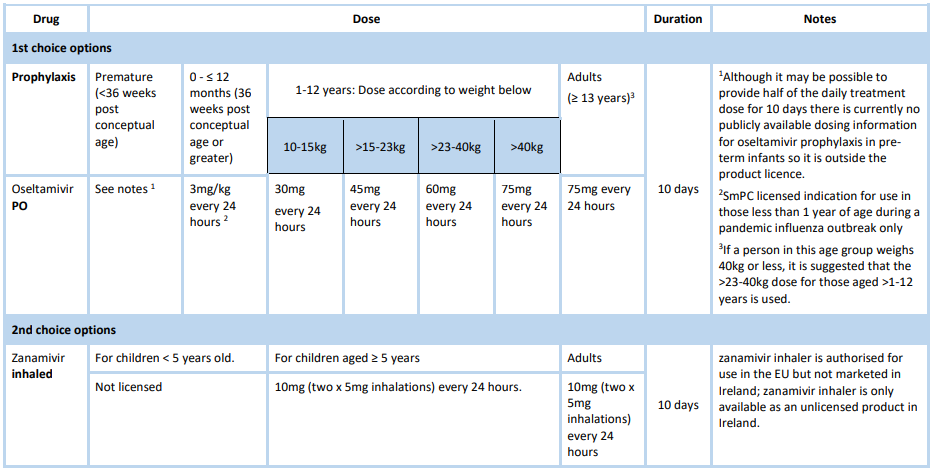
Chemoprophylaxis
Chemoprophylaxis should be reserved for those in at risk groups who have had recent close contact with a person with influenza or influenza-like illness in the same household or residential setting. Previous influenza vaccination does not preclude the use of post exposure prophylaxis, in particular where localised outbreaks occur in residential care facilities. For more information consult the Antiviral Treatment and Prophylaxis Guidance.
Antiviral dosage and schedules for chemoprophylaxis
back to top
Defined Risk Groups for Antivirals - high risk complicated influenza
- Age 65 years and over
- Pregnancy (including up to two weeks post partum), see HPSC Guidance link below.
- Children aged <2 years of age
- Chronic respiratory disease including those on medication for asthma
- Chronic heart, kidney, liver or neurological disease
- Diabetes mellitus
- Haemoglobinopathies
- Immunosuppression (whether due to treatment or disease e.g. HIV)
- Morbid obesity (BMI ≥40)
- Those with any condition that can compromise respiratory function (e.g. cognitive dysfunction, spinal cord injury, seizure, or other neuromuscular disorder), especially those attending special schools/day centres
- Persons with Down Syndrome
- Persons with moderate to severe neurodevelopmental disorders such as cerebral palsy and intellectual disability
- Residents of nursing homes or other residential care facilities
For more information consult the Antiviral Treatment and Prophylaxis Guidance.
Residential Care Facilities
Patient Information
Safe Prescribing
Back to top
Reviewed December 2022