This page provides a brief summary of the disease and the vaccine that is available to prevent it. Links to more detailed information are provided at the bottom of the page.
What is meningococcal disease?
Meningococcal disease is a serious illness caused by the bacteria Neisseria meningitidis. This bacterial infection can cause meningitis (inflammation of the lining around the brain) and septicaemia (blood poisoning).
There are different groups of the Neisseria meningitidis bacteria that cause meningococcal disease. Before the introduction of the meningitis C (MenC) vaccine in 2000, groups B and C caused most cases of meningococcal disease in Ireland. Thanks to the MenC vaccine against group C bacteria, the number of cases of meningococcal disease due to group C bacteria has fallen dramatically. Most cases are now caused by group B bacteria.
How do people get meningococcal disease?
Meningococcal bacteria can live at the back of the throat or in the nose. Most people, who carry these bacteria (carriers), remain well but they can spread the bacteria to others through coughing, sneezing, or kissing. Close personal contact with a carrier sometimes leads to infection. You need many hours of close personal contact to become infected as the bacteria do not survive long outside the body.
Meningococcal disease may occur at any age but the highest rate of meningococcal disease occurs in children under 5 years of age, especially children under one year of age. The next highest risk group are young people aged 15-19 years. In Ireland the risk of infection is highest in winter and early spring.
How long is the incubation period for meningococcal B disease?
A small minority of individuals who pick up N. meningitidis develop invasive infection after an incubation period which is typically 1-10 days, although usually less than four days.
How many cases of meningococcal disease occur in Ireland?
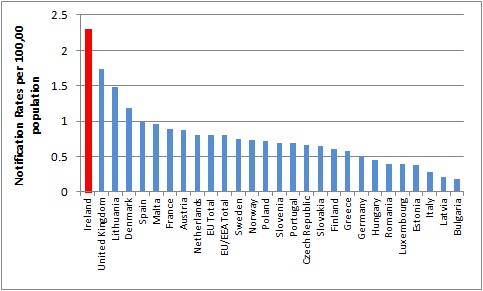
Ireland has one of the highest notification rates of invasive meningococcal disease (IMD) in Europe based on confirmed cases in the EU/EEA, 2008-2012 (Figure 1).
Figure 1. Rates of IMD, EU/EEA, 2008-2012
Source HPSC
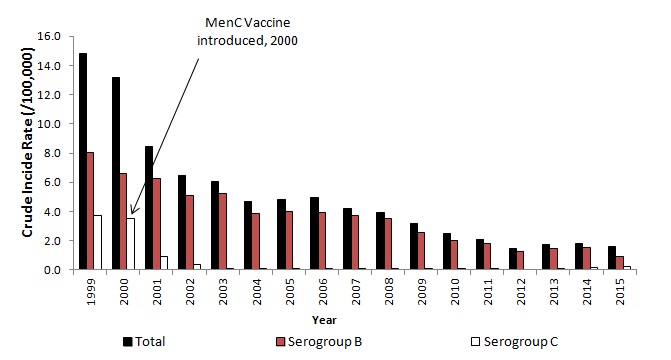
Since the introduction of the meningococcal C (MenC) vaccine, meningococcal B disease has been the predominant cause of invasive meningococcal disease in Ireland (Figure 2).
Figure 2. Crude incidence rate of IMD in Ireland, 1999-2015
Source HPSC
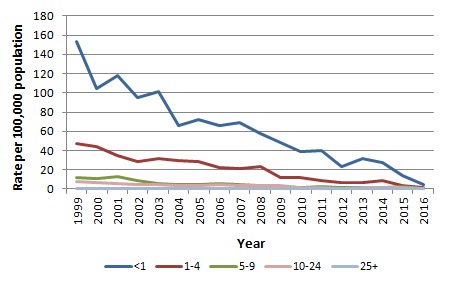
In Ireland, the rate of invasive meningococcal B disease has been steadily decreasing (Figure 3). In 2015 the age specific incidence rate (ASIR) among infants, less than one year of age, for meningococcal B disease was 13.8/100,000. In the 1-4 year age group it was 3.87/100,000. The case-fatality rate from invasive meningococcal B disease is less than 5%.
Figure 3. Crude incidence rate of invasive meningococcal B disease in Ireland, 1999-2015
Source HPSC (you will be directed to the HPSC website)
What are the symptoms of meningococcal disease?
The onset of meningococcal disease can be very quick. The symptoms of meningococcal disease include fever, stiff neck, headache, joint pains, and a rash. If you think your child or baby has signs of meningococcal disease get medical help immediately from your G.P. or nearest paediatric Emergency Department. In some cases acting quickly to get medical help can mean the difference between life and death.
Meningococcal disease is a very serious life threatening illness.
Of the people who get meningococcal disease:
- 1 in 20 will die
- 1 in 10 people who recover will have a major disability such as deafness, brain damage or loss of fingers, toes, hands, feet, arms or legs.
Early diagnosis leads to early treatment with antibiotics and a greater chance that the person will make a full recovery. Early diagnosis is the key so if you suspect that someone may have meningitis or septicaemia seek medical attention immediately.
More information is available at http://www.meningitis.org/ireland (you will be directed to the Meningitis.org website)
Who should get meningococcal B (MenB) vaccine?
Meningococcal B disease is prevented by vaccination.
All children born on or after 1 October 2016 will now be given MenB vaccine at 2 and 4 months of age with a booster dose of MenB vaccine given at 12 months.
Are there any reasons why MenB vaccine should not be given?
Contraindications
- Anaphylactic reaction to a previous dose of the vaccine
- Anaphylactic reaction to any constituent of the vaccine, including kanamycin and latex
Precautions
- In cases of acute severe febrile illness, immunisation should be deferred until the baby is well.
- If babies have a known coagulation defects, caution with administration should be taken and pressure should be applied to the vaccine site for 1-2 minutes after vaccination.
Can MenB vaccine be given with other vaccines?
Studies have now shown that MenB vaccine can be given at the same time as any of the other PCI vaccines.
Why is a ‘2+1’ schedule recommended and not a ‘3+1’ schedule as per the MenB (Bexsero) licensed information?
Studies from the UK have demonstrated that appropriate protection was provided prior to the peak incidence of invasive meningococcal disease (peak at 5 months of age) using a 2 dose primary schedule. A third booster dose at 12 months provides prevention for the toddler age groups. This schedule is used in the UK and has been endorsed by National Immunisation Advisory Committee (NIAC), in Ireland. (you will be directed to the HIQA website)
How is the vaccine presented?
Bexsero is presented as a single dose prefilled syringe with 2 needles.
The orange needle is 16mm x 25G.
The blue needle is 25mm x 23G.
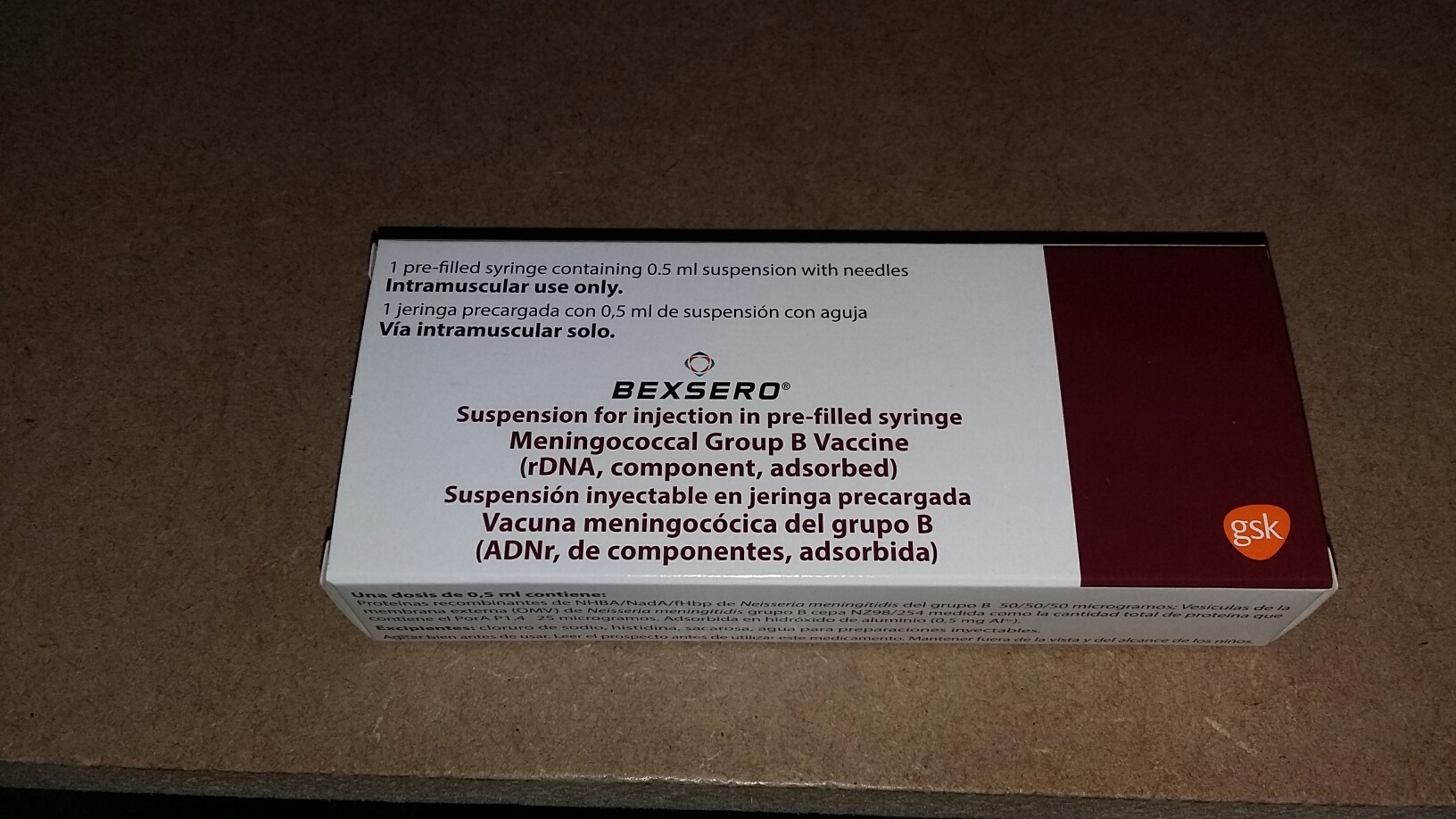
In accordance with the Immunisation Guidelines for Ireland the blue needle should be used as the orange needle is only indicated for babies less than 2.5-3kg. (you will be directed to the HIQA website)
Instructions for use
- Attach blue needle.
- If a fine off white deposit is observed in the syringe, shake to dispense.
The vaccine should be a homogeneous white opalascent liquid.
How is MenB vaccine administered?
MenB vaccine is given as an intramuscular injection into the anterolateral thigh.
Back to top
What order should the PCI vaccines be given?
At 2 months
Men B vaccine should be given first into the LEFT anterolateral thigh.
Then 6 in 1 vaccine followed by PCV should be given into the RIGHT anterolateral thigh.
This allows monitoring of any local adverse reactions and the most painful (PCV) is given last.
At 4 months
Men B vaccine should be given first into the LEFT anterolateral thigh.
Then 6 in1 vaccine should be given into the RIGHT anterolateral thigh.
At 12 months
Men B vaccine should be given first into the LEFT anterolateral thigh.
Then MMR vaccine should be given into the RIGHT anterolateral thigh.
What is the minimum age a baby can have the MenB vaccine?
The minimum age a baby can receive MenB vaccine is 6 weeks.
Back to top
What is the minimum interval between doses of vaccine?
The minimum interval between Dose 1 and 2 is 4 weeks.
The minimum interval between Dose 2 and 3 is 8 weeks.
What if a baby is late starting the vaccine schedule?
A baby born on or after 1st October 2016 can commence the MenB vaccine schedule at any stage, after 2 months of age as per the table below.
What if a baby is late starting the vaccine schedule?
|
Age
|
Number of doses
|
Schedule
|
|
3 - <10 months
|
3 doses
|
2 doses 2 months apart
3rd dose at ≥ 12 months
> 2 months after dose 2
|
|
10 - <2 years
|
2 doses
|
2 months apart
|
|
2 years
and older
|
2 doses
|
1 month apart
|
There is no MenB catch-up programme for children born prior to 1st October 2016.
Why is the meningococcal B (MenB) vaccine being introduced into the primary childhood immunisation schedule?
Meningococcal B infection is most common in infants under the age of 1 year. The Meningitis B vaccine only became available in 2013. The HSE received funding this year to include the MenB vaccine in the Primary Childhood Immunisation Schedule. All children born on or after 1 October 2016, will be given MenC vaccine at 6 months, rather than 4 months so that the MenB vaccine can be given at 2 and 4 months when children require protection from meningitis B infection.
Does the meningococcal B (MenB) vaccine protect against all meningococcal disease?
Only meningococcal B infection is prevented by the MenB vaccine. Other types of meningococcal infection are not covered by this vaccine.
Meningococcal C vaccine for protection against meningitis C infection has been given to children in Ireland since 2000. Since the vaccine was introduced in late 2000, the number of cases of meningococcal disease, due to group meningococcal group C bacteria, has declined dramatically. The number of reported cases has fallen from 139 in 2000 to just 6 in 2014, a reduction of 96%
It is very important to remain alert for symptoms of meningococcal disease as not all types are covered by the vaccines. Urgent medical attention should be sought if symptoms occur.
What are the side effects of MenB vaccine?
MenB vaccine is well tolerated by most babies and has a very good safety profile. The following are side effects which have been noted with this vaccine. Please see patient information leaflet (PIL) for full details available at www.hpra.ie.
Very Common (1 in 10 babies)
- Fever (>38oC)
- Tenderness / pain at injection site
- Skin rash
- Irritable
- Vomiting / diarrhoea
- Unusual crying
Uncommon (1 in 100 to 1 in 1000 babies)
- High fever (>40oC)
- Seizures (including febrile seizures)
- Eczema
Rare (1 in 1,000 to 1 in 10,000 babies)
Kawasaki’s disease
Paracetamol after MenB vaccine
Paracetamol after MenB vaccine
|
When MenB vaccine is given with the other PCI vaccines there is a higher risk that the baby will develop a fever.
NIAC has recommended that babies are given liquid infant paracetamol at their 2 and 4 month (MenB) vaccinations to reduce the fever.
|
What is the pattern of fever expected after MenB vaccine?
Ibuprofen is not recommended.
Fever after MenB vaccine follows a predictable pattern. The fever rises over the first 6 hours and peaks at this point. It then decreases over 24 hours, when most fevers will be gone. By 36 hours post vaccination very few babies would be expected to still have a persisting fever.
Evidence has shown that if parents are told about the possibility of fever and give paracetamol (most particularly the first dose) then very few parents medically present because of their baby’s fever.
What strength of paracetamol should parents buy to give to their babies?
Parents should only buy liquid infant paracetamol which is suitable for babies.
The correct strength of liquid infant paracetamol for babies is 120mg/5ml - this is written on the front of the box and on the bottle label.
How much paracetamol should be given to babies?
Babies at their 2 and 4 month vaccine should receive 2.5ml (60mg) of liquid infant paracetamol.
How much paracetamol should be given to babies?
|
Liquid infant paracetamol
(120mg / 5 ml)
|
Dosage
|
2 month visit
|
4 month visit
|
|
Dose 1
|
2.5ml
|
At the time of injection
|
At the time of injection
|
|
Dose 2
|
2.5ml
|
4-6 hours after dose 1
|
4-6 hours after dose 1
|
|
Dose 3
|
2.5ml
|
4-6 hours after dose 2
|
4-6 hours after dose 2
|
Back to top
How many doses and how often should paracetamol be given?
Babies should receive 3 doses (2.5ml) of liquid infant paracetamol (120mg / 5ml) after their 2 and 4 month visits. The first dose (2.5ml) should be given at or just after the vaccine is given. Dose 2 (2.5ml) should be given 4-6 hours later and dose 3 (2.5ml) should be given a further 4-6 hours after dose 2.
What about babies who present late for vaccination?
Paracetamol should be given to babies up to 12 months of age after their Men B vaccines if given with other childhood vaccines.
What dosage of paracetamol should be given to very small babies?
If a baby weighs less than 3.5kg (7lb 7oz) at their 6-week check, the baby should be re-weighed at the time of vaccination.
If they weigh less than 4kg (8lb 8oz), paracetamol should be given at a dosage of 15mg/kg.
Will paracetamol stop babies getting a fever?
No, it reduces the rate of fever by approximately 50%. Therefore parents should be informed that their baby might still get a fever, despite giving them paracetamol. However, it is likely that it will be a lower fever and shorter lived if they give paracetamol than if they do not give paracetamol to their baby.
Back to top
What if a baby still has a fever after the 3 doses of paracetamol have been given?
If the fever continues and the baby is well an additional dose of paracetamol may be given 4-6 hours after dose 3 i.e. a total of 4 doses of paracetamol in the first 24 hours after vaccination.
If the baby is unwell at any stage or has a fever (>39oC) outside of the 4 doses of liquid infant paracetamol, they should be medically reviewed.
Should paracetamol be given before vaccination?
It is important that babies are not given paracetamol prior to vaccination, as the GP or practice nurse will need to be able to assess that the baby is well to receive the vaccines.
Should paracetamol be given at the 12 month visit?
Paracetamol does NOT need to be routinely given at the 12 month visit for MenB vaccine, as a babies’ risk of fever after the vaccine at this age is no different from the risk of fever after any other PCI vaccines.
What does the paracetamol patient information leaflet (PIL) say?
The PIL states that babies aged 2-3 months should not be given more than 2 doses of paracetamol.
As stated in the meningococcal chapter of the Immunisation guidelines for Ireland, where advice differs from the PIL or Summary of Product Characteristics (SmPC) the recommendations based on current expert advice from NIAC should be followed.
Back to top
Why has the advice about paracetamol changed?
Up until now the routine use of paracetamol for pain and fever after vaccinations was not recommended. This was because there was a study that showed a possible interference with the development of an appropriate immune response to PCV vaccine.
Further evidence has now shown that there is no evidence of a decrease in the immune response when paracetamol is given with MenB vaccine and the other primary childhood immunisations.
Should paracetamol be given to a baby who is late starting their MenB vaccines?
Paracetamol should be given when babies under 12 months receive MenB vaccine with other PCI vaccines.
Should paracetamol be given to a baby was born before 37 weeks?
The SmPC for paracetamol states it is contraindicated to give paracetamol to babies born before 37 weeks. However, as per NIAC guidelines paracetamol can and should be given to babies born before 37 weeks using a dose correct for their weight. The dose is calculated as above for babies weighing less than 4kgs (8lb 8oz).
What should I do if a baby requires MenB vaccine at 6 or 7 weeks of age, when the SmPC for paracetamol states paracetamol should not be given to babies less than 2 months of age?
Paracetamol should be given to babies from 6 weeks of age using a dose correct for their weight. The dose is calculated as above for babies weighing less than 4kgs (8lb 8oz).
Back to top
What if a baby was born prematurely?
Babies born prematurely should still receive their MenB vaccinations at the appropriate chronological age.
What if a baby has already had a confirmed meningococcal B infection?
MenB vaccine is recommended for cases of meningococcal B who have not previously received MenB vaccine.
What if a baby is immunosuppressed?
There is no contraindication to MenB vaccine in immunosuppressed babies.
How does meningococcal B (MenB) vaccine work?
MenB vaccine contains extracts from the meningococcal B bacteria. The vaccine works by making the body's immune system respond to the bacteria, without causing disease.
How effective is MenB vaccine?
MenB vaccine has been shown to very effective at producing an immune response to the particular strains of meningococcal B causing invasive meningococcal disease in Ireland. Research has suggested that up to 88% of meningococcal B strains should be protected by MenB vaccine.
Back to top
What is in MenB vaccine?
A full list of vaccine constituents can be found at www.hpra.ie (you will be directed to the HPRA website)
Are other countries vaccinating against MenB?
There are many countries with recommendations for MenB vaccine including UK, USA, Italy, Austria and Spain. However the UK and Italy (12 regions) are the only countries offering a national funded immunisation programme for MenB vaccine, as is being provided now in Ireland.
What about cases and contacts of MenB disease?
MenB vaccine should be given to cases and all previously unimmunised close contacts of a case of any age and to control outbreaks of meningococcal disease as agreed by the Department of Public Health and the Health Protection Surveillance Centre.
What age group can MenB vaccine be given to?
MenB vaccine is indicated for individuals from 2 months and older. There is no upper age limit. The minimum age at which Bexsero can be given is 6 weeks
Back to top
What vaccine schedule should be used for cases and contacts?
The schedule ranges from 2‐3 doses depending on the age as per the table below.
|
Age
|
Number of doses
|
Schedule
|
|
2 - <10 months
|
3 doses
|
2 doses 2 months apart and booster at 12 months
|
|
10 - <12 months
|
2 doses
|
1 dose
and
booster at 12 months or older, 2 months after the first dose
|
|
12 months
and older
|
2 doses
|
2 doses 2 months apart
|
Back to top
What are the side effects in older children and adults?
Infants and children up to 10 years
Very common (1/10):
Fever (≥38ºC) loss of appetite, tenderness or pain at the injection site, skin rash, sleepiness, irritability, unusual crying, vomiting, diarrhoea
Uncommon (1/1000, <1/100):
High fever (≥40°C), seizures (including febrile seizures), eczema
Rare (1/10,000, <1/1000):
Kawasaki disease
From 11 years and older
Very common (1/10):
Tenderness or pain at the injection site, headache, nausea, myalgia, arthralgia
Back to top
Should paracetamol be given to cases and contacts?
Paracetamol does NOT need to be given routinely to cases and contacts who are 12 months and older.
See advice here for children less than 12 months of age
Can MenB vaccine be administered during pregnancy?
Yes. There is no evidence of risk from vaccinating pregnant women with inactivated bacterial vaccines so MenB vaccine can be given to pregnant women
Can MenB vaccine be given to breastfeeding mothers?
Yes. There is no evidence of risk from vaccinating breastfeeding mothers with inactivated bacterial vaccines so MenB vaccine can be given to breastfeeding mothers.
Where can I find out more?
You can ask for further information regarding immunisation from your G.P., Public Health Nurse or local health office.
In addition the links below provide some more detailed information:
This page was updated on 28 January 2019