Building a Better Health Service
Keep track of our progress

Tallaght University Hospital celebrates Staff Hero Awards
“These exceptional individuals were nominated by their colleagues for showing outstanding dedication and truly embodying our core vision of 'People Caring for People to Live Better Lives,” according to John Kelly, Tallaght University Hospital (TUH) Interim CEO, speaking as he announced the winners of the hospital’s Annual Heroes Awards 2025. “I want to commend each of them sincerely for the very significant impact they have had on our hospital, our patients, and their colleagues."

Ollie the therapy dog brings joy at University Hospital Galway
“His little presence has made such a huge difference to the children, cheering them up and assisting in calming those who are anxious about being in hospital - he’s an amazing dog,” according to Tracey Torpey, Clinical Skills Facilitator, Paediatric Unit, University Hospital Galway (UHG) explaining the impact their therapy dog has had.
New patient identification initiative launched at Tallaght Hospital
Tallaght University Hospital (TUH) have launched an innovative animated short film to help with patient care. Called ‘Expect to be Checked’ the production is the outcome of what they consider to be the first Positive Patient IDentification (PPID) committee nationally.

Patients to benefit from cardiac rehabilitation in West and North West
“As someone who has directly benefited from cardiac rehabilitation, I know how vital these services are. Expanding and improving access means more patients will get the support they need to recover and stay well,” according to Denis Goggin, welcomed the recently announced expansion of Cardiac Rehabilitation Services in the HSE West and North West.

New Tallaght hospital research supports men who have had testicular cancer
“This new research paper provides a practical solution to issues faced by men who have been treated for testicular cancer,” according to Ray McDermott, Clinical Professor of Medical Oncology at Tallaght University Hospital (TUH) and Clinical Lead at Cancer Trials Ireland.

Galway brothers undergo same life-saving heart procedure
University Hospital Galway (UHG) achieved a significant milestone in cardiac care in recent months by becoming the first hospital in Ireland and the UK to implant the Avalus Ultra biological aortic valve, a major advancement in the field of valvular heart surgery.

Waterford hospital app helps children prepare for tests and procedures
An App designed for children due for admission to University Hospital Waterford has been launched in recent weeks. Paediatric and Theatre Department Nurse Specialists in University Hospital Waterford (UHW) have been working with a company called Little Journey (UK) to develop a platform for children.

Cormac’s health journey raises awareness about penile cancer
“I didn’t know penile cancer existed and I’m sure there are many others who didn’t know either,” explains Cormac France, outlining his diagnosis and treatment journey and encouraging others to get themselves checked: “I paid a big price for not knowing. I was almost at the point of no return, where chemo or radiotherapy wouldn’t have worked. I had an operation in University Hospital Waterford before Christmas but if I had left it until after Christmas I was told I wouldn’t have seen March.

Carndonagh Day Care Centre Services at heart of local community
“The Carndonagh Day Care Centre for Older Persons provides much needed vital service to our community,” according to Tony Doherty, Chairperson for the Restoration of Carndonagh Day Care Services Committee, speaking in recent weeks as a special event was held to mark the official re-opening of Carndonagh Day Care Centre.

Marina and Deneka help enhance patient experience at Roscommon Hospital
“Our Advanced Nurse Practitioner-led service is truly transformative – it significantly enhances the patient experience and delivers measurable improvements in both access to care and clinical outcomes. We’re delighted to welcome Deneka and Marina to our expanding team,” according to Ursula Morgan, Director of Nursing at Roscommon University Hospital (RUH).

“Nourishing with care” at Waterford Festival of Food
The programme for this year’s staging of one of Ireland’s largest and longest-running community food festivals featured a demonstration and discussion on innovative dining practices currently being developed at the HSE’s Dungarvan Community Hospital.

Roscommon hospital works with local GAA to promote skin cancer awareness
“Approximately 1,290 people are diagnosed with melanoma skin cancer each year in Ireland,” according to Bernie Finneran, Advanced Nurse Practitioner in Skin Cancer at Roscommon University Hospital. Bernie was highlighting the importance of sun protection, as Roscommon University Hospital donated sunscreen dispensers to Roscommon GAA – Roscommon Gaels and Dr Hyde Park - to help raise awareness around skin cancer prevention.

Remote monitoring initiative helps Paul manage chronic conditions
Five years ago, Paul Wallace’s life changed after emergency surgery revealed not just spinal issues, but that his heart was functioning at only 10%. With intensive treatment, his heart function improved to 40%, but managing multiple chronic conditions became his new reality.

HSE encourages people to consider rewarding Health Care Assistant career
“Health Care Assistants (HCAs) constitute 14% of the health service workforce and work across many sectors. They are an integral part of the multidisciplinary teams and contribute towards providing care of the highest quality, whilst showing respect, kindness, consideration, and empathy in their communication and interaction with their patients,” according to Anne Marie Hoey, HSE Chief People Officer, commending the good work done by Health Care Assistants across the country.

Patients at Sligo hospitals benefit from new developments
“These new developments are very welcome additions to the hospital,” according to Grainne McCann, Manager, HSE Sligo University Hospital (SUH), speaking as the Minister for Health, Jennifer Carroll MacNeill performed two official openings and turned a sod on new 42 bed ward block during a recent visit.

First brick laid for new Carrick on Shannon Community Hospital
“This new Community Hospital represents a major enhancement in patient care in our region and will ensure that older people in our communities can receive specialist, person-centred care closer to their home,” according to John Fitzmaurice, HSE IHA Manager for Sligo Leitrim, speaking as the first brick of the new 90 bedded Community Hospital in Carrick on Shannon, Co Leitrim was recently laid by Minister for Health Jennifer Carroll MacNeill.

Tallaght University Hospital study to benefit patients
A new study at Tallaght University Hospital (TUH) has discovered an innovative solution which helps patients complete a particular type of diagnostic procedure - capsule endoscopy. Considered the gold standard, capsule endoscopy is a procedure used to examine the small intestine.

Midlands Limb Clinic initiative improving patient care
“I am delighted to support this valuable initiative which has proven to reduce the reoccurrence of leg ulcers and further reduce the need for hospital based services,” according to Mr Madhavan, Vascular Surgeon based in the Midland Regional Hospital Tullamore and St James’s Hospital, Dublin, speaking about a new Vascular Outreach Integrated Care Clinic.

New Tullamore Hospital preoperative guidance enhances patient comfort
Summary Sip Til Send, a new approach to preoperative drinking for patients in the Midlands Regional Hospital Tullamore, (MRHT), has enhanced patient comfort, according to Allison Burke, Trauma Coordinator, MRHT. “The new guidance aims to make patients more comfortable before their procedures, but also provides benefits afterwards too. We know from patient feedback that having to go for prolonged periods of time without being able to drink is uncomfortable and causes dehydration.”

Integrated Hip and Knee Pathway Reduces Need for Surgery
Summary “We’re seeing excellent outcomes and a huge improvement in the quality of life of our patients who have participated in this programme,” according to Mr Alan Walsh Consultant Orthopaedic Surgeon, speaking about the HSE National Osteoarthritis (OA) Hip and Knee Pathway which has had a life-changing impact on patients, improving their mobility and quality of life. Piloted in Navan and Waterford, the Hip and Knee Pathway has already served over 1,700 people suffering with osteoarthritis.

First patients seen in new HSE Kilkenny facility for older people
“Welcoming patients at Oriel House marks a significant achievement - allowing us to deliver integrated care for older people with complex needs while also helping them maintain independence and live well at home,” according to Majella Cunningham, Operational Lead for the HSE Carlow Kilkenny Integrated Care Programme for Older Persons Programme as they opened their doors for the first time in recent weeks.

Tallaght University Hospital first to pioneer Advanced Lab Testing Technology
“The installation of this advanced piece of equipment marks a significant step forward for the service,” according to John Kelly, Interim CEO of Tallaght University Hospital (TUH), welcoming the introduction of pioneering advanced technology which will speed up and significantly increase the hospital’s capacity to test patient samples.

New Cork Endometriosis Centre to provide specialist care for women
“The launch of the centre is a significant step toward improving access to specialised healthcare and addressing the long-standing gaps in endometriosis care. This is an exciting time for women’s health, and I am proud that CUMH is the leading the way,” according to Dr Mairead O’ Riordan, Consultant Obstetrician and Gynaecologist and Clinical Director, Cork University Maternity Hospital (CUMH), speaking as the Minister for Health, Jennifer Carroll MacNeill opened a new HSE Supra-Regional Endometriosis Centre to improve access to care for women with advanced endometriosis.

Joe’s positive experience encourages others to accept bowel screening invitation
“I’m alive and kicking thanks to a letter I got from BowelScreen. I don’t want people to be afraid of getting screened for bowel cancer - it’s a first step. If it comes back negative, happy days. If there is an issue - deal with it. Don’t let it go,” according to Joe Grogan from Tuam, Co Galway, who is encouraging those people in the 59 – 70 age group to avail of free bowel screening.

Roscommon University Hospital appoints Advanced Nurse Practitioner in Respiratory Care
“It's wonderful to see that the service is already making a significant difference in the lives of our patients,” according to Sarah Daly who was recently appointed to the role of Advanced Nurse Practitioner (ANP) in Roscommon University Hospital (RUH).

Winners of the first national GreenTech in healthcare announced
“We are delighted to be involved in this recent Greentech in healthcare call and initiative with Health Innovation Hub Ireland (HIHI) and the Irish College of GPs,” according to Dr Philip Crowley HSE National Director Climate and Global Health, speaking as the winners of its GreenTech in healthcare call were announced in recent days.

Role of the Health Care Assistant valued
“The role of the Health Care Assistant is to generally support our registered nurses and registered midwives in the delivery of safe and effective healthcare,” according to Dr Patrick Glackin, Area Director of Nursing and Midwifery Planning and Development HSE West. “There are approximately 22,000 Health Care Assistants in Ireland. Primarily they work across our acute hospital services, mental health services and older persons services.

HSE new Health App praised in first weeks after launch
“The launch of the first version of the new HSE Health App represents the next step forward in our digital transformation journey, as we seek to harness the power of data and innovation to help improve access to care for patients and enhance efficiencies across services,” according to HSE CEO Bernard Gloster, speaking in recent weeks as the Department of Health and the HSE launched the first version Health App.

HSE project addresses links between smoking, HPV and cervical cancer
“We know that smoking is a risk factor for persistent HPV (the human papillomavirus) – that is HPV that your body cannot clear, which can lead to cell abnormalities in the cervix and cervical cancer,” according to Professor Nóirín Russell, CervicalCheck Clinical Director, HSE National Screening Service. “HPV is the cause of most cervical cancers. Most people will get HPV at some stage in their lives. For most people, their immune system will clear the virus naturally from their body within one to two years. For some people, the virus will remain active.”

“A Flake of My Soul” poetry collection launched by HSE supported writers’ group
experiencing a sense of accomplishment and pride for work done, the acceptance of good days and bad, and expressing curiosity and encouragement towards each other’s work,” according to Eileen Byrne, Senior Occupational Therapist, speaking about ‘A Flake of My Soul,’ a new collection of poetry produced through a creative writing project supported by HSE Waterford Mental Health Services in partnership with the Waterford Healing Arts programme and supported by Rethink Ireland and Creative Waterford.

Hereditary Haemochromatosis (HH) Education Programme to benefit patients
“We are delighted that more GP Practices will now be able to provide this care to their patients. It will potentially remove the need for patients to attend hospital to have a venesection procedure performed,” according to Dr Conor Mitchell, GP and HSE GP Lead for the Sligo/South Donegal Community Healthcare Network, speaking as a bespoke education programme for GP Practices around the treatment of Hereditary Haemochromatosis (HH) was being introduced in the North West.

Folklore project engages care centre residents in Enniscorthy
“The residents and staff love to see Michael come in. In between his visits, it’s a topic of conversation – residents try to keep in mind a little phrase or a story to relate or discuss upon the next engagement, according to Mary Fox, Nurse Activities Co-ordinator at St John’s Community Hospital (SJCH), Enniscorthy, speaking about the ongoing collection of folklore material in Co Wexford that is currently being featured in the activities programme of a HSE residential care centre in the county, involving artist and filmmaker Michael Fortune.

State of the art new mental health facility opened in Dungarvan
“Today is a landmark day for mental health services across Waterford and particularly here in Dungarvan and the west of the county,” according to Minister of State with responsibility for Mental Health, Mary Butler TD, speaking at the official opening of the new facility in recent weeks.

Event showcases Wexford Chronic Disease Programme
Visitors to the Enniscorthy Primary Care Centre on St Valentine’s Day this year may have noticed an event was happening on the day, where caring for the heart and other services associated with the HSE Integrated Care Programme for Chronic Disease (ICPCD) service for Co Wexford were being showcased.

Advanced Nurse Practitioner in Diabetes appointed at Roscommon University Hospital
“We have an excellent diabetes service here at Roscommon University Hospital, and to have two visiting Consultant Endocrinologists from Sligo and Galway University Hospitals aligned to the service and supporting us is fantastic,” according to Brid Ni Chlochartaigh, who was recently appointed to the Role of Advanced Nurse Practitioner (ANP) in Diabetes at the Roscommon hospital.

Midwives reflect on rewarding job experience
“My mum was a midwife and inspired me to want to do midwifery,” according to Orla Mongan, an Advanced Midwife Practitioner working in Wexford General Hospital. “When I was given the opportunity to train as a midwife I loved it from the moment I did it and more than 22 years later I still love it.”

HSE launches new first version Health App
“I found all the videos and pregnancy exercises really useful – the new HSE Health App explains what to do throughout the entire pregnancy, gives specific information related to the trimester you are in, what to expect – and it kept all my appointments in one place too,” according to Helene Troisannt who shared her experiences of using the newly launched HSE Health App during her second pregnancy at Cork University Maternity Hospital.

Kilkenny open-air activity programme aiming to combat cancer
“Participants aged over 50 years were invited to take part in physiotherapy-led one hour physical activity sessions, twice weekly in two pilot rounds during 2024,” according to Sinead Gavin, Physiotherapy Manager, HSE Community Services Carlow Kilkenny, speaking about the Urban Action Against Cancer (UcanACT) programme in Kilkenny.

Harbour Friends Programme helps those with Younger Onset Dementia live well
“I love coming here. I have had lots of happy times here with everyone,” according to Padraig Boland, member of the Harbour Friends Programme in South Dublin. “It’s one of the highlights of my week.”

HSE consent policy event a shared learning experience
Early last year the HSE launched the updated National Consent Policy. Since then the Easy to Read version of the policy has been updated and is available on hse.ie/nationalconsentpolicy.

Cardiac Rehabilitation initiative helping heart health
A new video developed by the HSE aimed at increasing the number of patients participating in a Cardiac Rehabilitation programme in Cork and Kerry has been positively received locally.

Orla encourages women to take up CervicalCheck appointment
“The bottom line is cervical screening prevents cervical cancer,” stresses Orla Loftus, Advanced Nurse Practitioner, Knock Medical Centre, Co Mayo. A registered sample taker with CervicalCheck, Ireland’s national cervical screening programme, Orla began her nursing career in 1997 when, she explains, “there was little awareness of cervical cancer and no national screening programme. The introduction of CervicalCheck – an evidence-based, high[1]quality programme - in 2008 was an uplifting moment.”
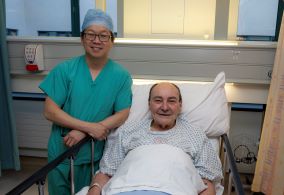
University Hospital Galway first public hospital to use innovative cardiac procedure
“This is a new beginning for me as I look forward to the future,” according to Galway resident, Sean Rankin, who recently became the first patient to undergo a ground-breaking procedure for treating atrial fibrillation (Afib) at University Hospital Galway. Sean, who had been suffering from atrial fibrillation for many years explained how he had sought treatment for his condition “following the success of my lung cancer surgery with Professor Soo. I’m doing very well and I am very pleased with the way everything went.”

Connie helping to reduce barriers to cervical screening
“Through my work in one of Ireland’s Sexual Assault Treatment Units (SATU) I provide responsive, holistic, patient-centred medical and forensic care, including on-site psychological support, for people aged 14 years and over who have experienced sexual violence,” explains Connie McGilloway, HSE Advanced Nurse Practitioner and Forensic Clinical Examiner, based in the SATU in Letterkenny, Co Donegal.
Innovative Social Farming initiative supports young people
“Social Farming was a really enjoyable experience – it opened my mind to alternative support measures that can help me,” according to Brendan Clifford, 14, one of the seven young people who got hands on experience so Social Farming in the HSE Mid West in recent months. “I looked forward to Wednesday afternoons with anticipation, and the two hours went by rapidly. My participation in Social Farming gave me great enjoyment, confidence, and pleasure.”

New HSE campaign to address HIV stigma in Ireland
“Like anyone else, with effective treatment, we can live long happy lives and there is zero chance that we can pass on HIV to our sexual partner. We have partnered with the HSE to let everyone know there is nothing shameful about living with HIV,” according to Robbie Lawlor, HIV activist, speaking as the HSE launched a new campaign addressing HIV stigma in Ireland.

Natasha seeks to demystify colposcopy process for women
Summary “I want to demystify the colposcopy process for women so they can feel more confident about it,” says Natasha Mahon, Clinical Midwife Manager (CMM2) and accredited colposcopist working at the National Maternity Hospital, Holles Street, Dublin. “I want to demystify the colposcopy process for women so they can feel more confident about it,” says Natasha Mahon, Clinical Midwife Manager (CMM2) and accredited colposcopist working at the National Maternity Hospital, Holles Street, Dublin.

Tipperary Horticultural Project praised for impact on clients
International guests were recently welcomed by the HSE South Tipperary Substance Misuse Team to the horticultural project it uses in Clonmel as part of recovery work with users of its service. Counterparts from Croatia, Spain and Slovenia, some of whom provide support to people with addictions through work on the land, were in Ireland as part of an EU Agri Next Exchange Programme, a project in which Tipperary Education and Training Board participate.

Sligo Memory Support Service helps Michael
“It was very helpful listening to other people talking openly and sincerely about their problems – it helped because I thought I was the only person with that particular problem,” according to Michael Kilcoyne who recently participated in a clinic led by the Memory Assessment Support Service (MASS) in Sligo. Michael explained how he had been “referred to the memory clinic by my GP.

Tipperary Sign Café promotes Irish Sign Language
A Sign Café is up and running in Tipperary, a resource that organisers and participants hope will be of significant value in the promotion of Irish Sign Language (ISL).

Donegal event highlights importance of safeguarding
“Safeguarding means putting measures in place to uphold the rights of citizens to support their health and wellbeing, to reduce their risk of harm, and to empower people to protect ourselves,” according to Edel Quinn, Head of Service for Disability Services Cavan, Donegal, Leitrim, Monaghan, Sligo, speaking as Donegal Disability Services recently held its second annual Safeguarding Awareness Event.

International recognition for Tipperary Garda and HSE Disability Services collaboration
A joint initiative, involving An Garda Síochána and the HSE Disability Services in Tipperary, has received international recognition in the 2024 Europol Excellence in Innovation Awards. The ‘Inside Out’ project in Tipperary Town aims to raise awareness of the impact of crime on vulnerable people, including people with disabilities and older persons.

Elaine outlines rewards of working as a Health Care Assistant
The main thing I love about being a HCA is the connection I have with the patients,” according to Elaine Clifford, Health Care Assistant, St James’s Hospital, Dublin. In her post almost 28 years, Elaine has been outlining how much she enjoys the role and in the interaction with the patients: “It means so much just knowing that during a very difficult time in their lives that you have made a difference and hopefully made things a little bit easier for them during their stay.”

HSE Wicklow Community Unit officially opened
“Service users and staff at the Wicklow Community Unit are witnessing the benefits of integration between the acute hospital and community healthcare sectors first-hand,” according to Martina Queally, Regional Executive Officer, HSE Dublin and South East, speaking at the recent official opening of Wicklow Community Unit in Wicklow Town.

Public Health Nursing – An opportunity to make a difference
“Becoming a Public Health Nurse was always on my radar,” according to Gemma Donegan, Public Health Nurse (PHN) based in the Primary Care Centre in Shannon, Co Clare. Prior to becoming a Public Health Nurse Gemma worked for four years as a midwife and for three years as a general nurse. Along with nursing colleagues across the country, Gemma is outlining how much she contributes in the work she does and also explains the rewards she experiences.

Successful first of its kind HSE and Climate and Health Alliance event
“Change is already taking place across many areas we identified in the HSE’s Climate Action Plan,” according to Dr Philip Crowley, National Director of Wellbeing, Equality, Climate and Global Health, HSE, speaking at the first of its kind conference involving the HSE’s Climate Action Programme and the Climate and Health Alliance in recent days

New Alzheimer Society Wexford Activity Lodge opened
“Every year, 10 to 15 people in Co Wexford are diagnosed with young onset dementia. We saw that there was a gap in services that they could access, as many such resources are focused on older people. The conversation that has borne fruit in the provision of the Activity Lodge included the voices of those with young onset dementia and identification by them of supports they required,” according to Sally Veale, a social worker with the HSE’s Memory Assessment Support Service, speaking as the new Alzheimer Society of Ireland’s Activity Lodge was officially opened in Enniscorthy, Co Wexford in recent weeks.

University Hospital Galway becomes first hospital nationally to use new biological aortic heart valve
“Already, I feel much more alive and energetic, and I can’t wait to get back to my usual daily routines of driving, farming, and walking my dog,” according to Noel O'Brien from Tynagh, County Galway who recently became the first patient to undergo Avalus Ultra valve implant surgery led by Professor Alan Soo and his surgical team at University Hospital Galway.

Kilkenny event highlights importance of safeguarding
“Without awareness raising there can be no recognition of abuse. That really is the first step,” according to Prof Eleanor Bantry White, addressing a recent gathering in Kilkenny marking National Safeguarding Day. Over 200 delegates attended the event organised by the HSE Safeguarding and Protection Team, Amber Women’s Refuge and the School of Applied Social Studies in University College Cork (UCC).

New Bereavement Support Initiative launched in Galway
“During grief, there are often difficult moments, where the bereaved may feel overwhelmed, confused, distressed and alone. Often the bereaved don’t have an awareness of the supports that are available to them in their local community that would be an invaluable support to them during this sad, sensitive and difficult time,” according to Vivian Roche-Fahy, Bereavement Liaison Officer at Galway University Hospitals.

Tallaght University Hospital celebrates four years at Age-Related Unit
“I am privileged to work with excellent colleagues who always put the patient’s needs at the centre of everything they do,” according to Dr Aoife Fallon, Consultant Geriatrician and Clinical Lead, speaking as Tallaght University Hospital (TUH) celebrated four years of service at their Medical Gerontology Age-Related Unit at Tymon North.

Minister opens Rainbow Lodge Children’s Respite Centre in Monaghan
“Respite care is such an important support to families who are caring for children with additional needs. HSE Disability Services were delighted to be allocated the additional funding required to progress the respite service that will be delivered at Rainbow Lodge,” according to Edel Quinn, Head of Service for Disability, HSE Community Services Cavan, Donegal, Leitrim, Monaghan, Sligo, speaking at the recent official opening of the facility.

Special awards ceremony for people with diabetes
“It is a privilege for us in healthcare professions to be able to help in whatever way we can, but the celebration tonight is an acknowledgment of the achievements of these incredible individuals who serve as role models to all of us,” according to Professor Tim O’Brien, Clinical Director of Medicine MCAN at Saolta Hospital Group.

Award for Tallaght University Hospital app that helps chronic pancreatitis patients
“We felt there had to be a quicker and easier way to communicate, so now, by using the Smart CP (Chronic Pancreatitis) app, patients can report their symptoms daily,” according to Marie Egan, Advanced Nurse Practitioner specialising in adrenal and pancreas diseases, Tallaght University Hospital (TUH).

Participants praise Parkinson’s Disease Awareness Event in Bray
“It lifts everybody. It really shows what can happen when you bring together people who are living with Parkinson’s Disease,” according to Gary Boyle, Patient Advocate, commenting on an event held recently in HSE Primary Care Services, Bray, Co Wicklow, to mark World Parkinson’s Day.

Kelsie grateful for breastfeeding support
“I am very grateful for the expert help and advice from Sheila in getting through the persistent issues I had with breastfeeding Halle,” explains mum Kelsie O’Mahony, from Schull, Co Cork, reflecting on her positive experience of the support she received from Sheila Lucey, West Cork based public health nurse and lactation consultant.

Tallaght University Hospital staff recognised in Annual Awards
“It was with absolute pleasure that I announced the recipients of the annual Tallaght University Hospital (TUH) Heroes Awards,” according to Lucy Nugent TUH CEO, speaking at an event to mark the Annual Awards. “Our recipients were nominated by their peers and some by patients - recognising that they go that extra mile, and live our vision of ‘People Caring for People to Live Better Lives.’ I would like to congratulate them all and acknowledge the contribution they make to our hospital, to the lives of our patients, and to their colleagues.”

Award-winning Waterford mural helps promote breastfeeding
HSE staff recently gathered at a Waterford award-winning mural to highlight the importance of breastfeeding. The ‘Annabelle and Billy’ mural in Ballybricken, completed by London-based French street artist Zabou, is on the gable end of Mulligan’s Pharmacy, at the junction of Ballybricken Green and St Patrick’s Terrace in the town.
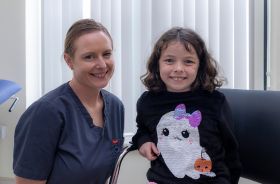
Siobháin appointed as Advanced Nurse Practitioner General Paediatric Care at Portiuncula Hospital
“I am very focused on delivering meaningful patient centred care. I want to ensure that patients who attend my service are empowered, educated and equipped to manage their conditions,” according to Siobháin Kiernan, who was recently appointed to the role of Advanced Nurse Practitioner in General Paediatric Integrated Care at Portiuncula University Hospital (PUH).

HSE raises awareness of sepsis at parkruns
“It was fantastic to see the number of people in pink at parkruns around the country helping to raise awareness of sepsis,” according to Denise McCarthy, ADON (Assistant Director of Nursing) Sepsis, HSE South South West Hospital Group, speaking of the initiative held recently to mark World Sepsis Day. “We know that early recognition and treatment of sepsis can lead to better outcomes so we wanted to include communities in our approach to increase knowledge around the signs and symptoms.”

Tallaght University Hospital doctor receives major European award
Tallaght University Hospital (TUH) based Dr Adam Dyer was recently presented with the Stefania Maggi Award at the 20th European Geriatric Medicine Society (EuGMS) Conference for new research which has implications for the detection of early Alzheimer's Disease.

Tallaght University Hospital Consultants give Free Health Talks to Public
Medical Consultants and other healthcare experts from Tallaght University Hospital (TUH) will give a series of free Health Talks for the public on a range of topics including Sepsis, Stroke, Menopause and How Patients can best Prepare for Surgery over the coming weeks. Coinciding with October Health Awareness Month in the Tallaght Community, all of the presentations will take place in the Conference Room at Tallaght County Library. All talks are free to attend.

Supporting marginalised women during pregnancy
Supporting Roma mothers during pregnancy and childbirth is of vital importance to the HSE. Many Roma women do not come forward for health checks early in their pregnancies due to a lack of awareness about the Irish healthcare system.

Nurses from across the South East gather to enhance care
“We are delighted that so many General Practice Nurses took time out of their busy practices to join us. Their commitment to advancing patient care and enhancing their skills in this vital area is truly commendable,” according to Liz Carroll, Professional Development Co-ordinator GP Nurses, HSE South East Community Healthcare, speaking as General Practice (GP) Nurses from Carlow, Kilkenny, Waterford, Wexford and South Tipperary came together for a Study Day hosted in Kilkenny recently.

Inis Mór Islanders complete Emergency First Responder Course
A group of volunteer islanders received Emergency First Responder Certificates and Emergency Services Driving Certificates from the HSE National Ambulance Service (NAS) at a special presentation held on Árainn/Inis Mór, off the coast of Galway, in recent weeks.

Cancer patients benefit from NCCP community cancer nursing eLearning programme
“I look forward to each and every appointment with my Community Intervention Team at my local primary care centre,” according to John Wall, a cancer patient in Ennis, sharing his experience of receiving cancer care in the community. “As strange as this may seem, this is simply because I now look forward to the chats and no longer fear the needles or whatever else may arise. The Community Intervention Teams around the country provide an absolutely invaluable service to patients and in so doing allow people like myself to avoid having to attend an acute hospital setting where possible.”

Clinicians working in partnership with patients to improve services
The HSE’s Clinical Lead in ePharmacy Brid Ryan is clear about the value of the feedback and insights brought by patient partners involved in the National e-Prescribing Project. Patient advocate Bernie O’Reilly, Patients for Patient Safety Ireland, and Pat Power, are two of the patient representatives on the project. They were among a number of patients to respond to an expression of interest circulated for patient representatives through the HSE National Patient Forum.

Minister visits HSE Waterford Substance Misuse Team
The HSE Substance Misuse Team in Waterford recently hosted Minister of State with responsibility for the National Drugs Strategy, Colm Burke TD, at their premises at St Otteran’s Hospital. The Minister was welcomed by Lisa Robson, Clinical Lead, Substance Misuse, HSE South East Community Healthcare. The Minister heard from various team members who provided an overview of their services. The Minister also heard from members of the local HSE Health and Wellbeing team.

Paralympian Monica's medals shine bright after 40 years
Forty years after USA president Ronald Reagan presented Cork’s Monica O’Kelly with her Paralympic silver medal, Monica gathered with her fellow residents at the HSE-run Farranlea Community Nursing Unit (CNU) to celebrate - and watch the 2024 Paralympics.

Positive TUH development with blood tests to detect Alzheimer’s Disease
Research just published in the leading international journal “Alzheimer’s Research and Therapy” outlines research on the effectiveness of a blood test in identifying the presence of Alzheimer’s disease. The research carried out in the Institute of Memory and Cognition at Tallaght University Hospital looked at the performance of a new blood test (plasma p-tau217) to detect the amyloid plaques that build up in the brain in people with Alzheimer's disease.

Galway University Hospitals introduce virtual care for COPD patients
“I have an excellent team of people behind me as well as the most wonderful nurse, who’s been very attentive with me. She’s gotten me through my infections at home on both occasions,” that’s according to Frank Galway patient Frank O'Connell, who was diagnosed with COPD nine years ago, and who was sharing his experience of virtual care in recent weeks. He described the profound impact being treated at home had on his daily life, explaining that “there’s an immense difference being treated at home as opposed to going into hospital. I hope the service is there for a long time to come, I really appreciate it.”

Galway clinical teams providing essential life-saving service
“This data shows the volume and complexity of trauma work that University Hospital Galway manages. Our clinical teams are providing an essential, life-saving service for the entire region while managing an increasing caseload,” according to Dr Alan Hussey, Clinical Director of Saolta’s Perioperative Directorate, who was reflecting on a study carried out by doctors at University Hospital Galway (UHG) which revealed the changing presentation of trauma patients, with a doubling of cases and older patient profile emerging over the course of a decade.

Roscommon Urology Clinic enhancing care for patients
The introduction of a Nurse-Led Urology Clinic at Roscommon University Hospital for Lower Urinary Tract Symptoms (LUTS) has significantly enhanced patient care, reduced waiting times, while also reducing the number of patients who require consultant urologist review.

Show season recognition for Haywood Lodge clients
“Colleagues throughout the mental health services in the South Tipperary area, whether in residential or community settings, prioritise the welfare of those we care for and support,” according to Aisling Carroll, Activities Co-ordinator, Haywood Lodge, speaking as residents and clients participated in and won prizes during recent Summer Show outings.

HSE National Patient Safety team win Innovation Award
The team behind the HSE National Patient Safety Alerts were recent winners of the National Innovation Award in the Best Design Led category at this year’s HSE Spark Summit. The Spark Innovation Programme seeks to support, promote and recognise innovation amongst healthcare staff within the HSE. The programme recognises the unique insights and perspectives of all frontline healthcare workers.

Rose praises COPD course that improved her health and daily life
“I used to have to go to the GP every couple of weeks. I got lots of chest infections, but I haven’t had one since I did the course,” according to Rose Brennan, from Dublin North Central, speaking about her experience of attending pulmonary rehabilitation – a specialised programme of exercise and education. Rose added that she was “very thankful for doing the course because it’s made a huge difference to my everyday life.”

TUH innovative projects benefit from HSE Spark Fund
“Projects such as Integrated Hand and Wrist Clinic are a perfect example of collaboration and exemplify our ability to deliver enhanced care in a hospital without walls to better the patient’s experience,” according to Head of Innovation at Tallaght University Hospital Natalie Cole, speaking after they received over €300,000 from the HSE’s Spark Impact Innovation Fund.

St John’s Hospital Sligo hosts Wise Roots festival
“The atmosphere was amazing through all areas of the hospital, with heart-warming scenes of residents and their families making memories and enjoying an afternoon of entertainment,” according to Nicola Scanlon Fox, Director of Nursing at St John’s Community Hospital reflecting on the inaugural Wise Roots age friendly festival held on the grounds of the facility in recent weeks.

Successful Open Day at Tullamore Ambulance Station
“The Open Day was a great opportunity for our staff to showcase their work,” according to Pat Mooney, General Manager, HSE National Ambulance Service (NAS) Midlands and Dublin Area, speaking after Tullamore Ambulance Station crews recently invited the local community to join them in learning about the life-saving work they carry out on a daily basis.

Kerry Men’s Health at the Mart event a success
"This has been a wonderful opportunity for men in Kerry to meet with a range of health and community professionals in a one-stop-shop; ask the questions, get the information, avail of the supports - and catch up with one another too,” according to Michelle Foley, Health Promotion and Improvement Officer with HSE Cork Kerry Community Healthcare, as she encouraged men in Kerry to take positives steps in minding their health in the weeks after the popular Men’s Health at the Mart event.

Will feels valued at HSE National Screening Service Public Patient Partnership Network
“The highlight of my Public Patient Partnership (PPP) experience so far has been realising that we PPP reps are valued and really listened to,” according to Will, who is encouraging others to join the PPP Network at the HSE National Screening Service (NSS). “You will see the changes that you have suggested whether it’s documents, leaflets, letters or processes – you know you are making a difference.”

HSE supported Kilkenny writing group launch poetry and prose book
A creative writing group established under the auspices of the HSE Mental Health Services supported Involvement Centre in Kilkenny has recently launched an anthology of poetry and prose. Entitled ‘White Twine and Old Suitcases’, the book was launched by Urlingford poet, writer and storyteller, Paddy Doyle. The publication was arranged by the HSE’s Recovery College South East, representing over a year’s work by 60 writers from the Co Kilkenny area.
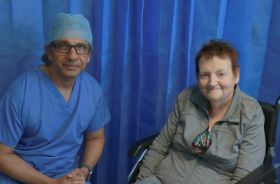
First patient in Ireland fitted with heart failure sensor at University Hospital Galway
University Hospital Galway (UHG) has become the first hospital in Ireland to introduce a new device designed to help patients suffering with heart failure better monitor their fluid levels in the body. Galway woman, Sheila Concannon from Spiddal, was the first patient in Ireland to be fitted with this early warning sensor which alerts medics to the patient’s condition worsening.

HSE Excellence Awards highlight innovation and success
Through its Excellence Awards, the HSE annually showcases staff innovation. The awards are an opportunity to celebrate examples of the great work that happens every day across the health service. They aim to encourage and inspire health service staff to develop and improve care and services for their patients, clients and communities. Applications are now open for the 2024 Health Service Excellence Awards. This year, there is a new category for projects focused on sustainability and climate action.

Paula delights in her role as Health Care Assistant
“I go home happy every day knowing that I’ve made somebody’s life a little bit better,” according to Paula Curran, Health Care Assistant working in the Day Centre for Older Persons in Clonskeagh Hospital, Dublin. Paula has been in her role for a number of years and really enjoys her work: “On average, on a weekly basis here, we would see between 75 to 80 clients – different people every day. Working as a Health Care Assistant I love making people feel happy. I love being able to do things for them. We work very well as a team here in Clonskeagh.”

Partnerships working to improve health and wellbeing of Tipperary Roma community
A variety of organisations are currently working together to improve the health and wellbeing of the Roma community in Co Tipperary. The HSE, Tipperary County Council and Youth Work Ireland Tipperary recently launched a needs assessment overview in addition to a report on the work of the Health and Accommodation Pilot Project that was established locally in 2022.

Valuable role of cancer support centres recognised
“This day marks a significant milestone in our collective efforts to support cancer patients and their families throughout Ireland. These centres provide invaluable psychosocial support and survivorship programmes, ensuring comprehensive care for those living with and beyond cancer,” according to Prof Risteárd Ó Laoide, National Director, HSE National Cancer Control Programme (NCCP). Prof Ó Laoide was speaking as the NCCP and Minister of State at the Department of Health, Colm Burke TD presented each member organisation of the NCCP Alliance with a plaque at a dedicated event.

Tallaght Hospital creates neurodiversity friendly environment in Emergency Department
In the evolving landscape of healthcare, ensuring inclusivity and accessibility for all patients is paramount. For neurodivergent individuals, navigating the high-stress environment of an Emergency Department (ED) can be particularly challenging. However, Tallaght University Hospital's ED is pioneering efforts to address these challenges, setting a standard for neurodiversity-friendly care.

New HSE storybook to support children bereaved by suicide
A new, free illustrated storybook ‘Safe Harbour’ that will support children who have been bereaved by suicide, is now available.

New state-of-the-art HSE drug trends laboratory opens
“This new laboratory means we can quickly and accurately identify dangerous substances in drug samples and detect emerging drug trends. Understanding what's happening in real-time allows us to offer relevant, effective support to people who use drugs. It also allows us to support healthcare professionals, making a significant difference in people's lives,” according to Bill Ebbitt, General Manager, HSE, at the National Drug Treatment Centre.

New women’s health café opens in Cork
“The Change Café is somewhere that people can come to and engage in an open and honest conversation,” according to Eleanor Moore, Principal Community Worker, Cork Kerry Community Healthcare.

Sligo University Hospital awarded second An Taisce Green Flag
Sligo University Hospital (SUH) recently received a second Green Flag from An Taisce. The internationally recognised Green Flag was awarded following a rigorous assessment process and recognises the commitment of hospital management and staff, HSE Estates, the NUI Galway Medical Academy and the hospital’s Green Campus Committee in developing the hospital as a healthcare facility that delivers high quality care and improved public health and wellbeing in an environmentally responsible and sustainable way.
