Comments from Expert Advisory Group
- The most common mammalian bites are associated with humans, dogs, and cats and infection is a risk if there is a break in the skin with bleeding.
- Seek specialist advice for bites from a wild or exotic animal (including birds) because the bacteria involved may be different and there may be a risk of other serious non-bacterial infections.
- Human bites are either clenched-fist injuries (‘fight bites’) or occlusal injuries. Most human bites occur on the hand. Human bites are known to be at risk of infection and subsequent complications with an infection rate ranging from 10-50%. Injuries to the clenched fist can be particularly serious.
- Dogs can cause significant crush injury and tissue devitalisation, in addition to laceration, puncture, and avulsion (tearing away of tissue) injury. An estimated 3% to 18% of dog bites become infected (median time to infection is 24 hours).
- Cats have weaker biting force than dogs however they have thin, sharp teeth; 85% of cat bite wounds are puncture wounds, which inoculate organisms deep into the tissue. Due to the high rate of puncture wounds, infection rates after cat bites may be as high as 50% (median time to infection is 12 hours). Infected cat bites are more likely to be associated with complications such as tenosynovitis, osteomyelitis and septic arthritis.
General Management Advice
- Assess and manage associated injuries (e.g. possible head injury / fractures etc.).
- Removal of any foreign bodies if applicable.
- The affected skin surface should be cleaned, and the unclosed wound copiously irrigated with warm running water, normal saline, or lactated Ringer's solution (with a giving set or IV catheter tip and large syringe).
- Assess tetanus and rabies risk (relevant travel).
- Assess HIV / hepatitis B & C risk (relevant to human bite) https://www.hpsc.ie/a-z/emi/
- Wound closure is a controversial issue. There is general agreement that infected wounds, and those seen more than 24 hours after the bite, should be left open.
- Consider referral to secondary care for facial injuries (except minor) or penetrating wounds involving underlying structures (joints, nerves, muscles, tendons, bones). Note: penetrating bites to the hands, feet or head are at particular risk of infection and complications.
- If the wound is infected, send pus or deep wound swab for culture.
- Antibiotics are recommended for infected bites (treatment) and, in certain circumstances, non-infected bites as prophylaxis.
Prophylaxis
Adopted from NICE NG184
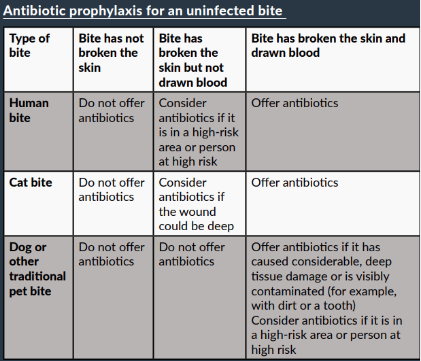
High-risk areas include the hands, feet, face, genitals, skin overlying cartilaginous structures or an area of poor circulation. People at high risk include those at risk of a serious wound infection because of a co-morbidity (such as diabetes, immunosuppression, asplenia/ impaired splenic function or decompensated liver disease).
Treatment
Offer an antibiotic (see Treatment table) for people with a human or animal bite if there are symptoms or signs of infection, such as increased pain, inflammation, fever, discharge or an unpleasant smell. If concerns regarding severe infection, e.g. septic arthritis, then seek specialist input. If systemically unwell, consider urgent referral as may rapidly progress and intravenous therapy may be required.
Reassess if:
- symptoms or signs of infection develop or worsen rapidly or significantly at any time
- the person becomes systemically unwell
- there is severe pain that is out of proportion to the infection
| BITES (HUMAN/CAT/DOG) ANTIBIOTIC TREATMENT TABLE |
| Drug |
Dose |
Duration* |
Notes |
| 1st choice option |
|
Co-amoxiclav
|
Adults:
625mg every 8 hours
Children:
See Paediatric dosing
|
3 days for prophylaxis
5 days for treatment*
|
Avoid in penicillin allergy.
|
| Adults: 2nd choice option / Penicillin Allergy |
|
Metronidazole
PLUS
|
400mg every 8 hours
|
3 days for prophylaxis
5 days for treatment*
|
Advise patients to avoid alcohol during metronidazole therapy and for at least 48 hours after stopping.
|
|
Doxycycline
|
200 mg on first day, then 100 mg or 200 mg every 24 hours
|
3 days for prophylaxis
5 days for treatment*
|
Contraindicated in pregnancy.
Advise to take with a glass of water andsit upright for 30 minutes after taking.
Absorption of doxycycline significantly impaired by antacids, iron/ calcium/ magnesium/ zinc-containing products and should be separated by at least 3 hours.
Risk of photosensitivity.
|
| Children aged 3 months and older: 2nd choice option / Penicillin Allergy |
|
|
|
|
|
|
Co-trimoxazole**
|
24mg/kg$ every 12 hours
Or
dose banding as below:
3 months - < 6 months: 120mg every 12 hours
≥6 months– < 6 years: 240mg every 12 hours
≥6-< 12 years: 480mg every 12 hours
≥12 years: 960mg every 12 hours (usual adult dose)
|
3 days for prophylaxis
5 days for treatment*
|
$Co-trimoxazole is a fixed combination of sulfamethoxazole and trimethoprim, 5:1 ratio.
Co-trimoxazole is associated with rare but serious side effects.
Discontinue immediately if rash develops.
Not recommended in < 6 weeks.
Avoid in blood disorders / G6PD deficiency.
|
| Pregnant women with Penicillin Allergy |
| Seek advice from Microbiologist |
*Note: A 5-day course is appropriate for treating most human or animal bites. The antibiotic course duration can be increased to 7 days if there was significant tissue destruction or the wound has penetrated bone, joint, tendon or vascular structures. Such wounds would require surgical management
** Co-trimoxazole dosing adopted from Paediatric Formulary Children’s Health Ireland (CHI) (Accessed Feb 2024)
Patient Information
Give advice to people with a human or animal bite about:
- possible adverse effects of antibiotics (if they have been offered antibiotics)
- seeking medical help if symptoms or signs of infection develop or worsen rapidly or significantly at any time, or do not start to improve within 24 to 48 hours of starting treatment.
Reviewed April 2024
